PLOS ONE welcomes RIAT (Restoring Invisible and Abandoned Trials) Initiative
Last week BMJ published an article proposing a new initiative aimed at solving an age-old problem in medical publishing: that of the perennial failure of investigators and sponsors to publish all of the results of all their trials, accurately and transparently. The BMJ paper ups the ante and invites independent, “restorative authors” to step in and take charge of unpublished or misreported trials. It’s not so much “publish and be damned” as “publish or be published”. Restorative authors can now use as their data source the documentation from a trial (often many thousands of pages of protocols, clinical study reports, and individual patient datasets or analysed datasets) obtained through freedom of information requests. The RIAT proposers (Peter Doshi, Kay Dickersin, David Healy, S Swaroop Vedula and Tom Jefferson) describe in their table 1 the many documents and datasets they have already obtained for a large number of trials, and which they’re willing to share. Doshi et al invite collaborators to their enterprise:
“We call on others to join us, to contribute trial documents they have obtained from public sources that need publishing or republishing, and to help us with the writing. We need volunteers to act in place of those who should have but did not make trial reports visible and accessible.”
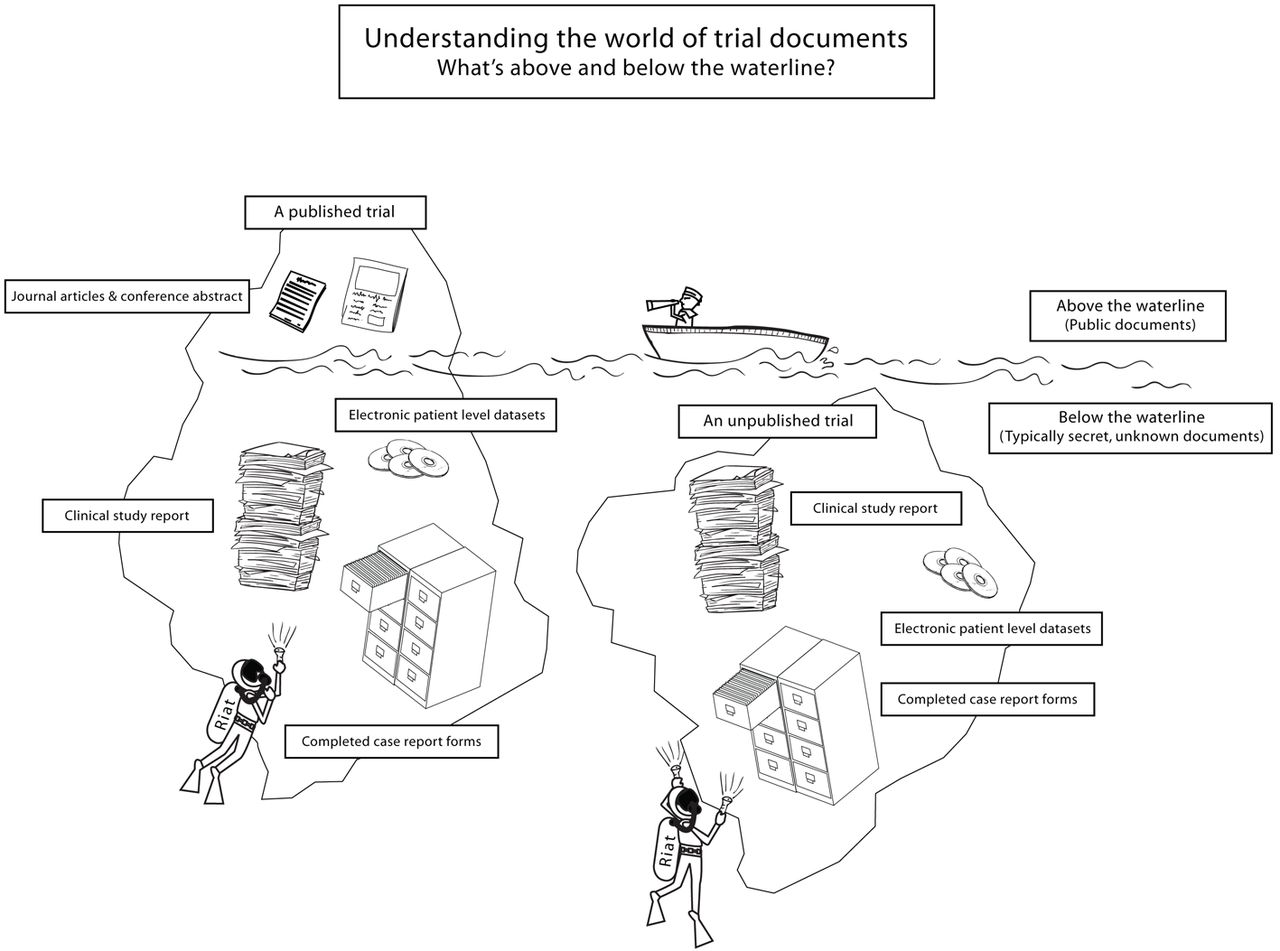
Image credit: Doshi et al, BMJ 2013;346:f2865 (Copyright CC BY-NC 3.0)
The initiative has already garnered support from our sister journal PLOS Medicine, which has co-signed an editorial announcing that they “commit to publishing restorative clinical trial submissions”. A detailed blog by PLOS Medicine editors discusses some of the hurdles that might need to be overcome for journals to publish restored trials by independent authors. For example, many “restored trials” may not have been originally registered in public trial registries, and restorative authors may have limited ability to establish whether the trials they are restoring were ethically conducted. Generally journals will only publish the results of trials which were publicly registered, and for which authors can take responsibility for ethical oversight and provide assurance to editors that their trial was carried out ethically. The PLOS Medicine editors invite feedback via their blog on these, and other issues.
The RIAT initiative is entirely concordant with PLOS ONE’s editorial aims and mission, which seeks to publish the results of all correctly reported, scientifically sound studies, irrespective of impact or the direction of results. PLOS ONE’s publication criteria do not discriminate against “negative results”, and the journal welcomes submission of re-analyses or replications of prior work, as well as analyses based on publicly available datasets. We do require that authors adhere to study-type-specific community standards for reporting, such as the CONSORT guidelines for reporting randomized trials (which has been adapted by the RIAT authors into a specific modification, the “RIATAR” tool for documenting the RIAT process).
Consequently, PLOS ONE now invites RIAT authors to consider submitting their “restored” trial reports to PLOS ONE for publication. These papers will be considered in the context of our existing publication criteria and editorial policies, although the editors are actively considering how the specific issues noted above in relation to trial registration and ethical integrity might best be interpreted. We ask RIAT authors to clearly identify when submitting (ideally in their cover letter) that they are responding to the RIAT initiative and are submitting a RIAT study. Authors should show that they have given the original triallists and sponsors an opportunity to publish their own study. They should do this by stating in their article methods section when they contacted the original triallists and sponsors, and by what date no response had been received. (If original triallists/sponsors have indicated they wish to restore their own trial, the RIAT proposal allows a “grace” period of a year, in which they should be allowed to publish without being scooped). We will also check Rapid Responses to the BMJ article to find out whether sponsors intend to restore their own trial. We also suggest that RIAT authors ensure they fully describe the methods that they have used to conduct RIAT, including the approaches to obtaining the datasets from the original trial, to reanalyze those data and through which they have come to their conclusions. This is particularly important if the trial has already been reported by the original triallists, and if RIAT authors are drawing different inferences based on a different approach to analysis. RIAT authors should take care to distinguish responsibility for the work they have done (in obtaining public data and conducting their reanalysis) from the work done by the original triallists (the conduct of the original trial), thereby establishing what authorship means in the context of a RIAT study.
Finally, the PLOS ONE editors have noted that PLOS ONE study is included in the list of clinical study reports amassed by the RIAT group (see Table 1, “Novartis FLUAD study, cited as reference 103 in the BMJ paper. PLOS ONE reference is given below). PLOS ONE is committed to correcting the publication record where necessary and therefore we will be in touch with the RIAT group to find out whether they can share with us any information on why this study is included in their list, and for what reasons a correction of the record may be needed. Quite separate from this, we also welcome any submission to PLOS ONE of a reanalysis of this trial from restorative authors, based on the clinical study reports that the RIAT group have obtained.
Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009;4:e4384
My conflict of interest declaration: I saw an earlier version of the RIAT article while it underwent peer review for BMJ, and provided some comments as part of the peer review process.
